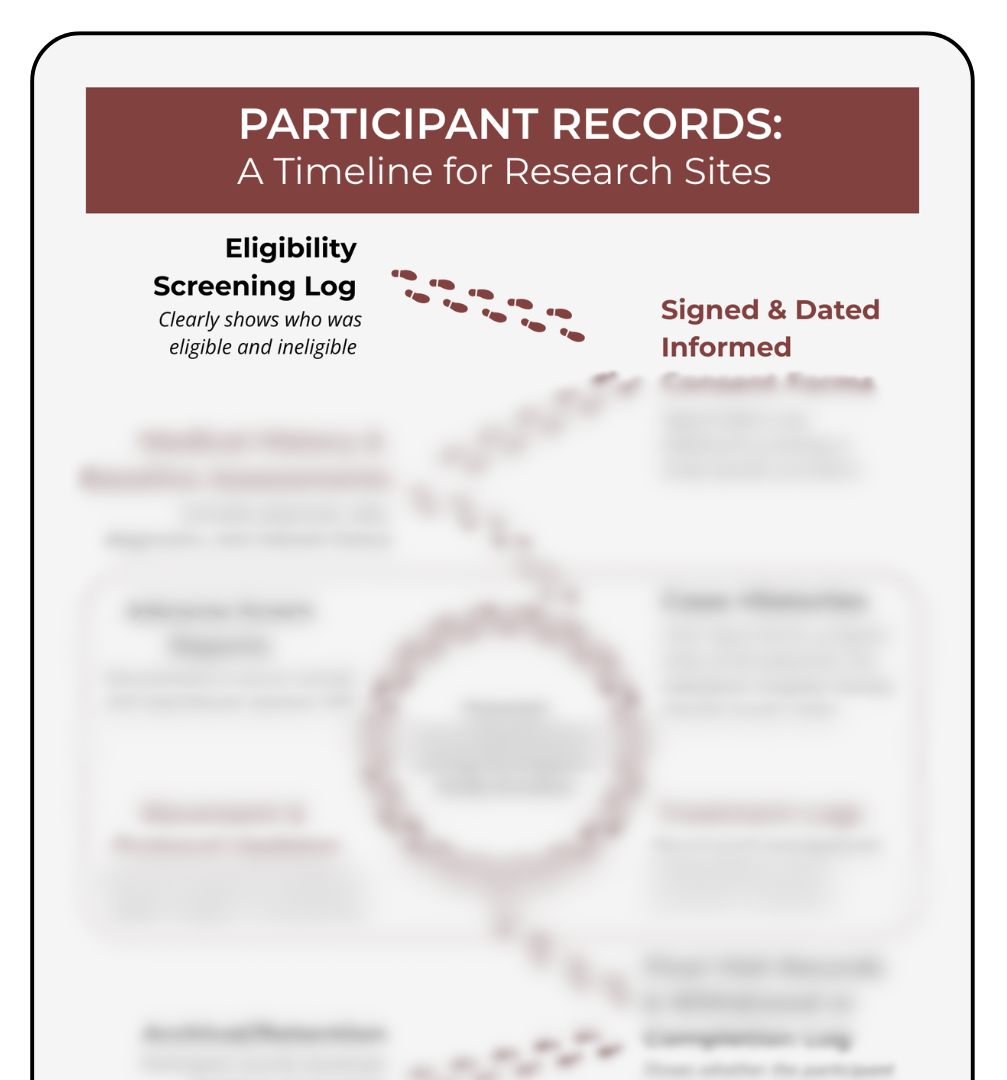
Participant documentation tells the full story of a study.
Participant documentation tells the full story of a study — and this guide makes it easier to understand that story from beginning to end. This timeline shows what records appear at screening, what continues throughout participation, and what must be finalized at completion or withdrawal. It includes essentials like eligibility assessments, consent forms, medical histories, treatment or procedure logs, visit notes, and AE reporting. Whether you’re onboarding new coordinators, teaching students, supporting investigators, or helping sponsors train sites, this visual guide clarifies how participant documentation fits together across the study. Use it during training, site startup, or day-to-day work to stay organized, compliant, and confident.
Know what to document and when. See which forms, logs, and assessments appear at screening, consent, study visits, safety events, and study close-out.
Make training easier for your team. Give new coordinators, students, and investigators a simple visual guide that clarifies the flow of participant documentation from start to finish.
Stay organized and inspection-ready. Use this overview to ensure participant records are complete, up to date, and easy to locate in source documents and regulatory binders.
Stay on track with participant documentation.
Your visual timeline for screening, consent, study visits, safety reporting, and long-term record retention — designed for new and experienced research teams alike.
$5.00