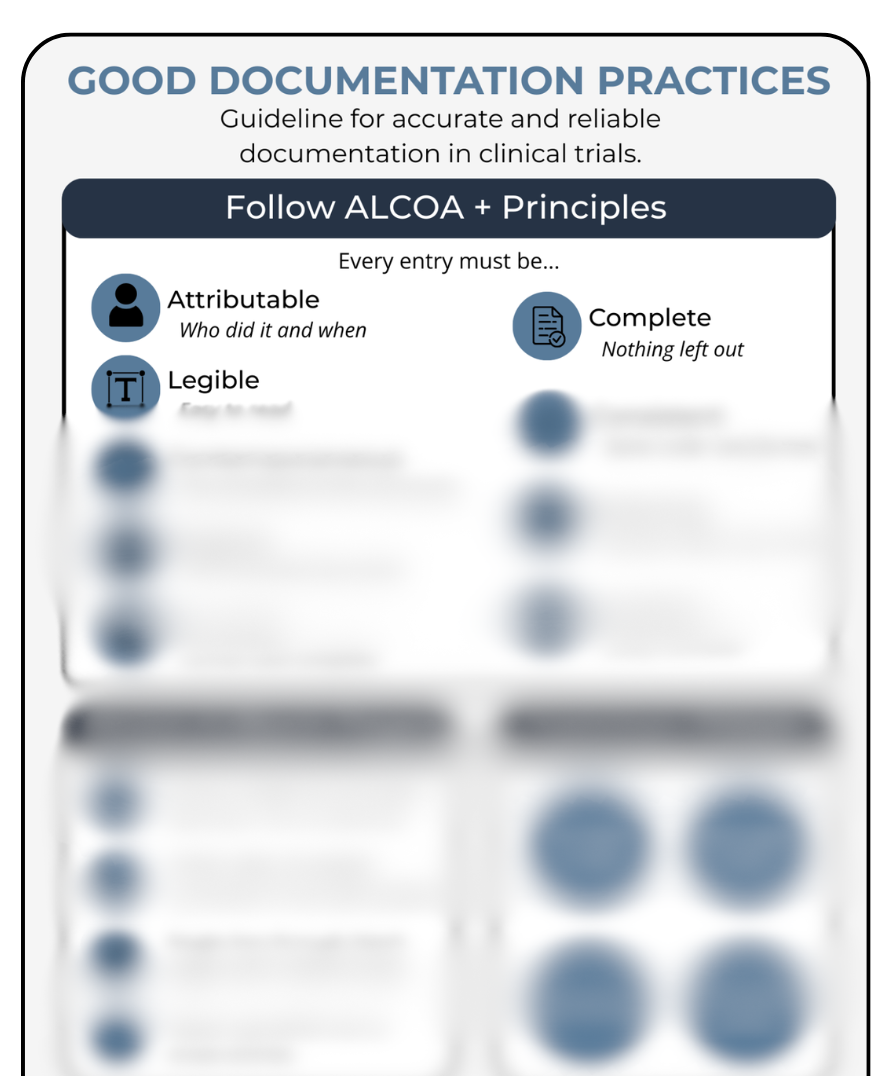
Write clear, compliant records. Understand exactly what makes each entry Attributable, Legible, Contemporaneous, Original, Accurate, Complete, and Consistent — the foundation of ALCOA+.
Avoid common documentation mistakes. Get simple reminders that help new staff, students, and busy teams prevent overwrites, missing dates, undocumented corrections, and other issues noted during audits and reviews.
Stay inspection-ready and build confidence. Use strong GDP habits that show sponsors, auditors, and regulators you take data quality seriously — complete, organized, and consistent every time.

The key to success.
Good Documentation Practices (GDP) form the backbone of credible research, and getting them right doesn’t have to feel complicated. This printable breaks down each of the ALCOA+ principles in plain language, so you can document research activities clearly, correctly, and confidently. Whether you’re new to clinical research, training a new coordinator, supporting students, refreshing your skills, or preparing for monitoring visits, this one-page guide helps you understand what every entry must be — Attributable, Legible, Contemporaneous, Original, Accurate, Complete, and Consistent. Perfect for onboarding packets, staff training, investigator support, and regulatory binders. Keep it nearby — you’ll use it more often than you expect.
Make documentation easier for yourself — and your team.
Your documentation tells the story of your research. This printable helps you get it right — clean, complete, and ready for review every time.
$5.00