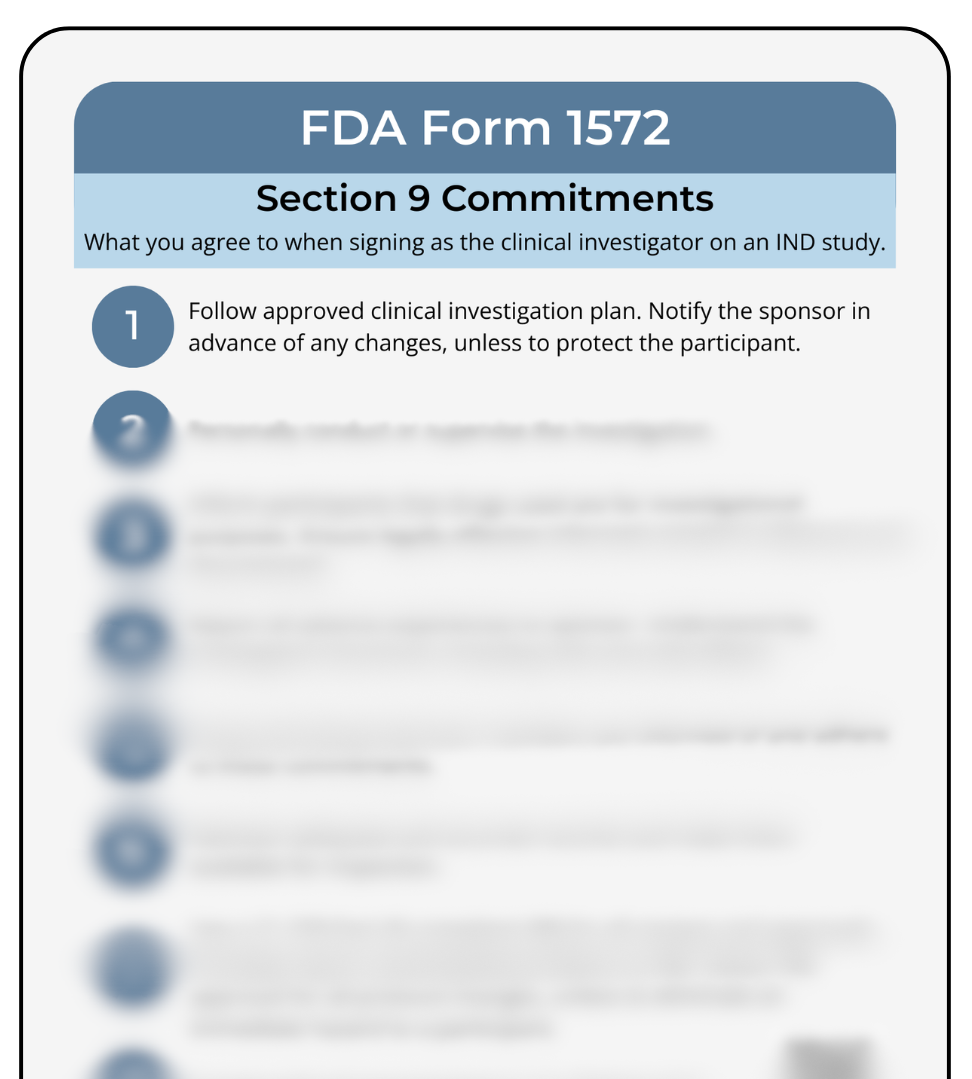
Have every Form 1572 commitment in one clear, accessible place.
When investigators sign the FDA Form 1572, they commit to a defined set of responsibilities that guide the conduct of the clinical investigation. This one-page reference pulls every Section 9 commitment into a clean, easy-to-review format so investigators, sponsors, coordinators, and students can quickly revisit what the FDA requires under Form 1572 and 21 CFR 312. Whether you’re onboarding a new investigator, supporting a study team, training students, or preparing for monitoring visits, this sheet helps everyone stay aligned on expectations around supervision, informed consent, IRB oversight, recordkeeping, protocol compliance, and safety reporting. It’s a practical addition to investigator packets, regulatory binders, sponsor training materials, and site onboarding workflows.

Why this quick sheet?
Understand your legal responsibilities.
Break down what the FDA requires when investigators sign Form 1572 — including adherence to the investigational plan, informed consent, accurate documentation, safety reporting, and more.
Support compliant, confident study conduct.
Use this reference to reinforce expectations around supervision, informed consent, safety reporting, IRB oversight, and recordkeeping — all grounded in 21 CFR 312 requirements.
Strengthen onboarding and communication across teams.
A practical tool for sponsors, investigators, and site staff to ensure everyone understands the same commitments the same way — consistent, transparent, and easy to reference anytime.
Keep responsibilities clear and accessible.
A straightforward reminder of the commitments tied to FDA Form 1572 — easy to reference, easy to share, and ready for real-world study work.
$5.00